A respirator fit test is a crucial step to help prevent disease transmission and keep your facilities compliant. Per OSHA, “Whenever respirators are required, employers must implement a written, worksite-specific respiratory protection program (RPP), including medical evaluation, fit testing, training, and other elements, as specified in OSHA’s Respiratory Protection standard (29 CFR 1910.134).”
Learn more about how to perform disposable respirator fit tests and the differences between qualitative and quantitative respirator fit mask testing.

Karl Seagren
Direct Supply Environmental Product Consultant & PPE Expert
Direct Supply Product Consultant Karl Seagren answers common questions about respirator and/or mask fit testing. Karl is Direct Supply’s subject matter expert for personal protective equipment (PPE) as well as other environmental products and topics, including infection prevention and safety. He regularly provides product training, keeps a pulse on the latest industry trends and evaluates new products. This includes the Evaclean disinfection and sanitizing system, which has become a key tool in the fight against COVID-19. Discover Karl’s insights into respiratory protection fit testing.
What is a respirator fit test?
A respirator fit test tests the seal between the respirator’s facepiece and your face. Respiratory protection fit testing can be performed to ensure tight fitting respirators and proper protection.
A person must perform a fit test with any respirator they will wear before initial use. It should only be performed after obtaining medical clearance. (Note: Someone with facial hair may not be able to achieve an adequate seal.) Then, they should perform a fit test at least annually or when any significant changes occur, such as weight gain or loss, facial surgery, or dental surgery. Perform a fit test with the same make, model, style and size of respirator that the person will wear when working.
If the make, model, style or size of respirator is changed, a new fit test should be performed. Additionally, if there are concerns about the fit based on the test, continue to test for a good seal with different respirator models, styles or sizes until an acceptable fit is identified. Document the results of your test.
Important: Respirator fit tests are not the same as user seal checks. That’s because a user seal check is a quick check performed by the wearer each time the respirator is put on to determine if the respirator is properly seated to the face or needs to be readjusted.
What’s the difference between qualitative vs quantitative fit test?
There are two types of respirator fit testing – a quantitative or qualitative respirator fit test. Qualitative tests are what OSHA recommends for healthcare environments. This pass/fail test uses sense of taste or smell in order to detect leakage in the respirator face piece. There are 4 types of approved qualitative tests:
- Saccharin (sweet)
- Bitrex (bitter)
- Isoamyl acetate (bananas)
- Irritant smoke (produces coughing)
When does testing need to be done?
OSHA requires respirator fit testing to be completed upon initial implementation of a Respiratory Protection Program and then once a year after that. OSHA has temporarily paused the need to do annual testing.
Is a fit test required for a N95 respirator?
Yes. Per OSHA guidance, if respirators are required, all tight-fitting, disposable and reusable respirators/masks should be fit tested. This includes N95 and surgical N95 respirators, which are common in Long Term Care. Other reusable and disposable respirators that should be fit tested include:
- N99
- N100
- R95
- P95
- P99
- P100
Any healthcare professional wearing reusable or disposable respirators should know how to perform respirator fit tests to properly wear respirators for optimal occupational safety.
Does the respirator fit test kit I use need to be made from the same manufacturer as my N95?
No. The brand does not matter when conducting reusable or disposable respirator fit testing. Any qualitative fit test kit should work with any brand of N95 respirator.
How long will a respirator fit test kit last?
Most sensitivity and test solutions for fit testing have a 3- to 5-year shelf life, if unopened. Once the ampules are opened, most need to be used within 4 hours.
Does Direct Supply offer respirator fit test kits?
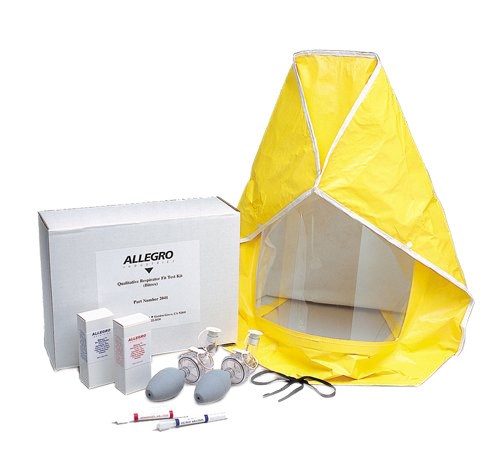
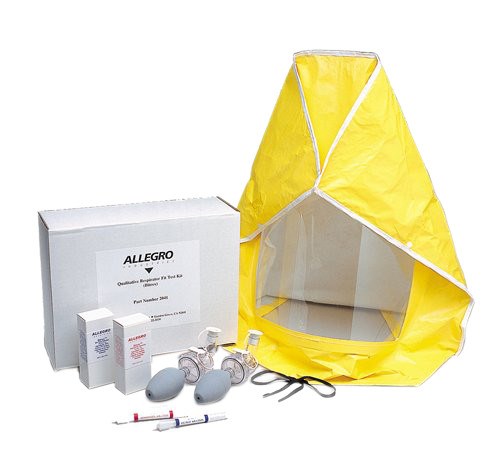
Yes! These two kits offer an OSHA compliant qualitative fit test for disposable and reusable respirators. New disposable glass ampules make pouring solution into nebulizers easier and reduce the chance of contamination. Each kit includes a test hood, sensitivity nebulizer, test nebulizer, 6 ampules of sensitivity solution and 6 ampules of test solution. Learn about the reusable and disposable respirator fit testing options below.
Allegro Bitrex Respirator Fit Test Kits create an unmistakable bitter taste that indicates breakthrough on the respirator fit.
Allegro Saccharin Respirator Fit Test Kits create an unmistakable sweet taste that indicates breakthrough on the respirator fit.
Each kit has enough solution (both sensitivity and test) to test 18 to 30 people (3 to 5 people per ampule of solution) Additional sensitivity and test solution can be purchased separate from the test kits. You can also find additional respirator fit test solution and an instructional video on how to perform the Allegro qualitative fit test.
What do I do if my staff aren’t passing their respirator fit tests?
Find out how many staff members are having trouble getting a good seal or knowing how to perform a respirator fit test. Not every respirator is going to work on every individual’s face size and shape. So ask if they’ve tried other models, styles and sizes. OSHA requires employers to offer different makes, models, shapes and sizes of respirators to employees until they find one that will fit. If you can’t find any that fit certain staff members, consider assigning those staff to non-COVID areas.
What’s the difference between an N95 and a surgical N95?
A surgical N95 is NIOSH-approved and also cleared by the FDA for use as a surgical mask. It’s recommended if the wearer will be exposed to liquids like sprays and splashes from things like suctioning and nebulizer treatments.
Does OSHA require respirator fit testing for KN95 respirators?
No. OSHA doesn’t currently require a respirator fit test of KN95s. These have the equivalent filtration capability to N95s, but normally come with ear loops rather than head straps. Some newer KN95s exist which have head straps. If you choose to fit test KN95s, review the most recent OSHA guidance with your legal team and keep it handy for reference.
Can I use an N95 that isn’t FDA approved?
Probably, if the N95 is NIOSH certified. For the duration of the public health emergency, the FDA has issued an EUA that allows healthcare workers to use NIOSH-approved respirators. You should make sure you are following any state or local guidelines, as well as the CDC’s guidance for conventional, contingency and crisis strategies for optimizing the supply of N95 respirators.
Have a question not answered here?
Contact your Direct Supply account manager or call 1-800-634-7328 and we’ll help you find the answer! We’re here to help you stay compliant with changing regulations and guidelines. We also carry a wide variety of infection prevention solutions to assist you, including: